After 11 years in the Coffer Lab, Cindy Fredericks is leaving us to join Niels Eijkelkamp’s and Onno Krannenburg’s Labs. Cindy has played an essential role in the work of numerous PhD students and postdocs in the lab and she will be sorely missed. We wish her all the best with the new challenges!
Paper alert: How does interferon-mediated intestinal damage drive T cell recruitment?
Cytokines like interferon-gamma are key players in decisions driving inflammation and regeneration. Using a 3D co-culture model based on human intestinal organoids, PhD students Alessandro Cutilli and Suze Jansen discovered that interferon-gamma can reprogram the gut lining, leading it to express a set of pro-inflammatory genes. Specifically, they found an upregulation of chemokines CXCL9, CXCL10, and CXCL11—molecules that are key to recruiting immune cells. Their findings also showed that this reprogramming enhances T-cell migration, largely driven by CXCL11. These insights could open new therapeutic avenues: targeting CXCL11 might help prevent T-cells from trafficking to the inflamed intestine, potentially offering relief for conditions like inflammatory bowel disease (IBD). Check out the full study at:
https://pubmed.ncbi.nlm.nih.gov/39302156/
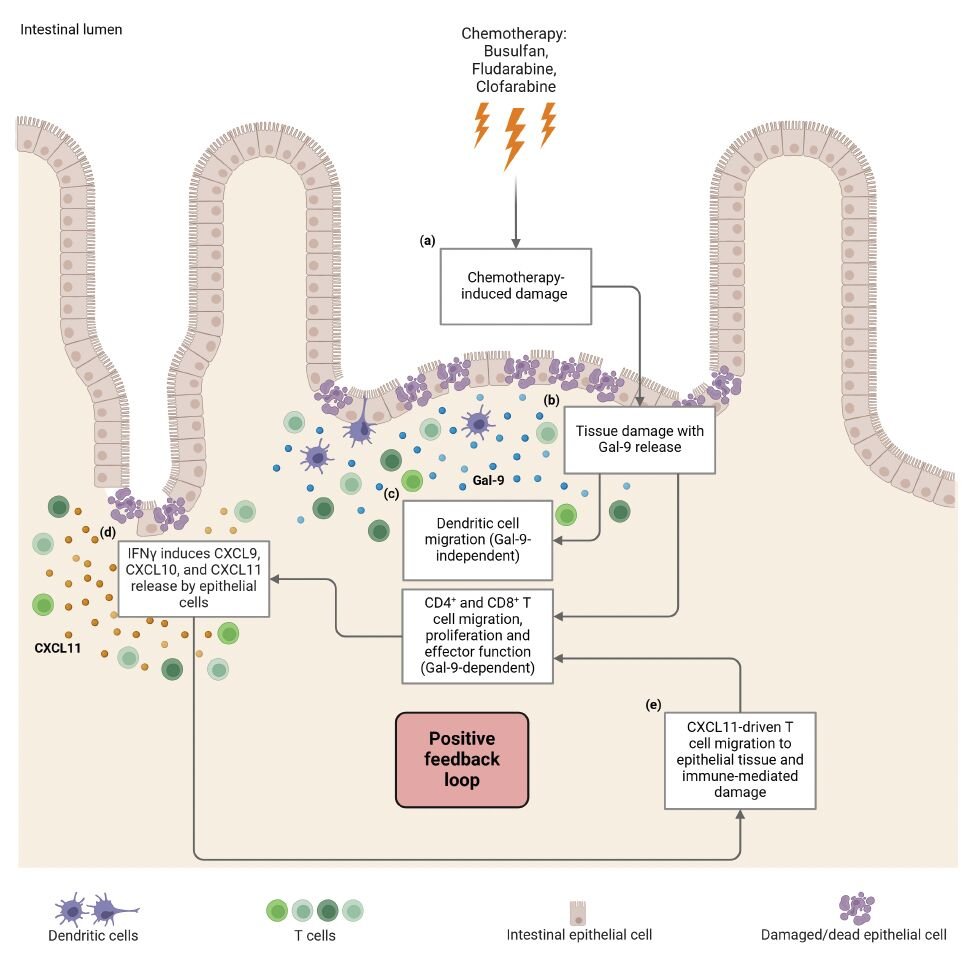
Paper alert: Chemotherapy induced intestinal damage directly modulates T cell behaviour
We have developed a human intestinal organoid-based 3D model system to study the direct effect of chemotherapy-induced intestinal epithelial cell (IEC) damage on T cell behavior. This has led to the identification of galactin-9 as playing an important role in driving CD4+ and CD8+ T cell responses. Congratulations to Suze Jansen and Alessandro Cutilli for all their work driving this study forward.
Highlights
Chemotherapy-induced intestinal epithelial damage can be modelled with organoids
3D-coculture model allows evaluation of epithelial damage on T cell homeostasis
Chemotherapy-induced epithelial damage modulates T cell activation and migration
Galectin-9 promotes T cell activation and migration in response to epithelial damage
Summary
The intestine is vulnerable to chemotherapy-induced damage due to the high rate of intestinal epithelial cell (IEC) proliferation. We have developed a human intestinal organoid-based 3D model system to study the direct effect of chemotherapy-induced IEC damage on T cell behavior. Exposure of intestinal organoids to busulfan, fludarabine, and clofarabine induced damage-related responses affecting both the capacity to regenerate and transcriptional reprogramming. In ex vivo co-culture assays, prior intestinal organoid damage resulted in increased T cell activation, proliferation, and migration. We identified galectin-9 (Gal-9) as a key molecule released by damaged organoids. The use of anti-Gal-9 blocking antibodies or CRISPR/Cas9-mediated Gal-9 knock-out prevented intestinal organoid damage-induced T cell proliferation, interferon-gamma release, and migration. Increased levels of Gal-9 were found early after HSCT chemotherapeutic conditioning in the plasma of patients who later developed acute GVHD. Taken together, chemotherapy-induced intestinal damage can influence T cell behavior in a Gal-9-dependent manner which may provide novel strategies for therapeutic intervention.
Welcome Claudia!
Cláudia Leite graduated in Biochemistry and holds a PhD in Molecular and Cell Biology from the University of Porto. During her PhD, she investigated how mitochondria adjusts their activity to regulate cell cycle progression in budding yeast. She has recently joined the Coffer lab as a postdoctoral researcher to investigate the role of nuclear acetyl-CoA-producing enzymes in regulating transcriptional reprograming.
Lab day out: canoeing in Holland's beautiful 'green heart'
CLICK PHOTO TO VIEW FULL SIZE
Paper: Nuclear metabolism controls chromatin remodelling during T cell activation
Extremely happy to have our study exploring nuclear metabolism in the control of chromatin remodeling published (link below). This became a huge project driven by Enric Mocholi and wouldn't have been possible without generous collaborations from groups in the EU, USA and Canada (through lockdown). A BIG thank you to all involved.
In brief
After T cell activation, histone acetylation and transcriptional reprogramming require glycolysis and the pyruvate dehydrogenase (PDH)-dependent production of extramitochondrial acetyl-CoA. Here we show that PDH translocates to the nucleus close to chromatin-remodeling complexes, highlighting how metabolic and histone-modifying enzymes cooperate in regulating T cell activation.
Highlights
- PDH is required for histone acetylation and transcription after T cell activation
- MPC1 and ACLY are not required for T cell activation and transcriptional reprogramming
- T cell activation leads to PDH nuclear translocation close to chromatin-remodeling complexes
Lab Jeu de Boules night out
The Winners!
Congratulations Dr. Anita Govers!
Many congratulations to Dr. Anita Govers who successfully defended her thesis "Epigenetic regulation of normal and aberrant myelopoiesis: a balancing act". It has been a long journey and super happy that we could celebrate this milestone together with co-promoter Dr. Marije Bartels. Many thanks to the thesis committee Prof. Marry van den Heuval-Eibrink, Prof. Femke van Wijk, Prof. Jurgen Kuball, Prof. Roland Kuiper, Prof. Joop Jansen en Dr. Marc Bierings.
In the last two decades, epigenetic changes have become more and more evident as a contributing factor in aberrant myelopoiesis, however the exact roles are yet poorly understood. Furthermore, knowledge about epigenetic regulation of normal hematopoiesis is far from complete. Nonetheless, the use of chromatin modifying drugs such as HDACi, has rapidly increased in the past years.
Work in this thesis is aimed to increase understanding the effects of epigenetic regulation and epigenetic modifiers on both normal and aberrant hematopoiesis.