We have developed a human intestinal organoid-based 3D model system to study the direct effect of chemotherapy-induced intestinal epithelial cell (IEC) damage on T cell behavior. This has led to the identification of galactin-9 as playing an important role in driving CD4+ and CD8+ T cell responses. Congratulations to Suze Jansen and Alessandro Cutilli for all their work driving this study forward.
Highlights
Chemotherapy-induced intestinal epithelial damage can be modelled with organoids
3D-coculture model allows evaluation of epithelial damage on T cell homeostasis
Chemotherapy-induced epithelial damage modulates T cell activation and migration
Galectin-9 promotes T cell activation and migration in response to epithelial damage
Summary
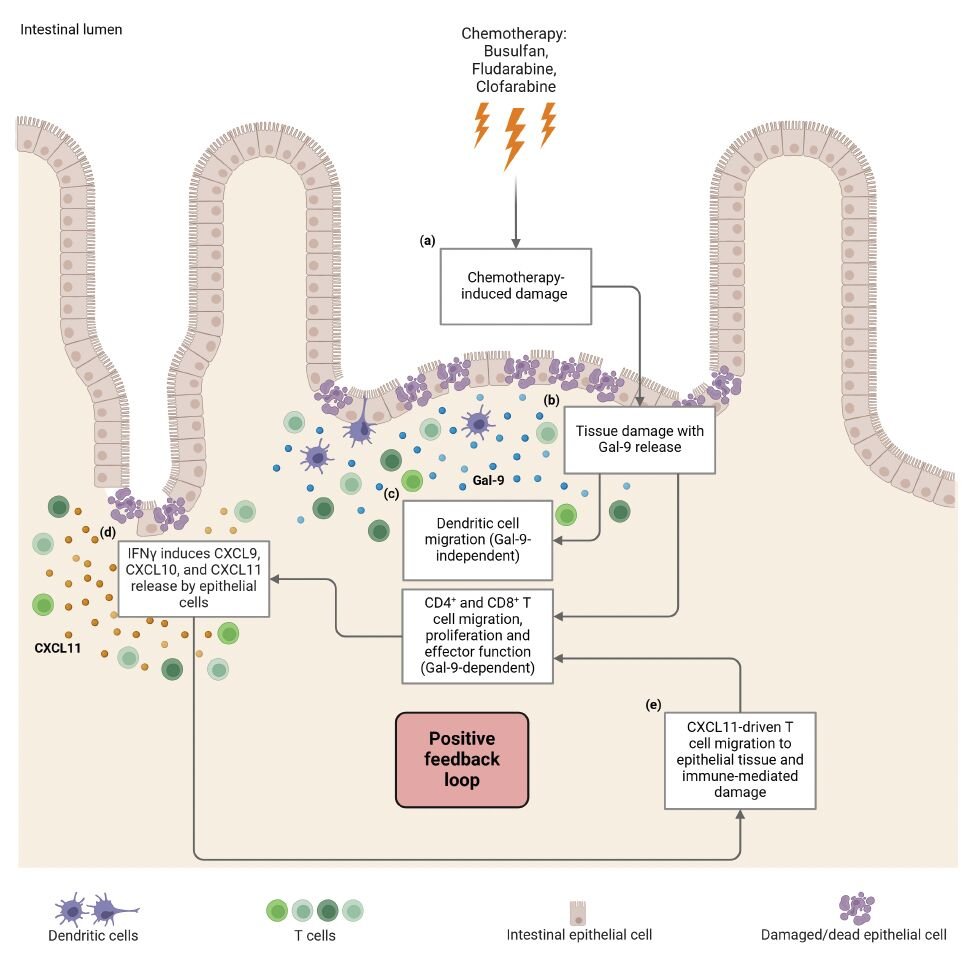
The intestine is vulnerable to chemotherapy-induced damage due to the high rate of intestinal epithelial cell (IEC) proliferation. We have developed a human intestinal organoid-based 3D model system to study the direct effect of chemotherapy-induced IEC damage on T cell behavior. Exposure of intestinal organoids to busulfan, fludarabine, and clofarabine induced damage-related responses affecting both the capacity to regenerate and transcriptional reprogramming. In ex vivo co-culture assays, prior intestinal organoid damage resulted in increased T cell activation, proliferation, and migration. We identified galectin-9 (Gal-9) as a key molecule released by damaged organoids. The use of anti-Gal-9 blocking antibodies or CRISPR/Cas9-mediated Gal-9 knock-out prevented intestinal organoid damage-induced T cell proliferation, interferon-gamma release, and migration. Increased levels of Gal-9 were found early after HSCT chemotherapeutic conditioning in the plasma of patients who later developed acute GVHD. Taken together, chemotherapy-induced intestinal damage can influence T cell behavior in a Gal-9-dependent manner which may provide novel strategies for therapeutic intervention.
Chemotherapy-induced intestinal epithelial damage directly promotes galectin-9-driven modulation of T cell behavior. Suze A. Jansen# ,Alessandro Cutilli#, Coco de Koning, Marliek van Hoesel,Cynthia L. Frederiks, Leire Saiz Sierra,Stefan Nierkens, Michal Mokry, Edward E.S. Nieuwenhuis,Alan M. Hanash, Enric Mocholi, Paul J. Coffer* and Caroline A. Lindemans*