Roukens et al Oncogene 2021
An underlying aspect of cellular plasticity in tumorigenesis is the re-activation of developmental pathways by tumor cells. Perhaps the best characterized is epithelial-to-mesenchymal-transition (EMT) where epithelial cells lose their tight junctions and gain migratory and invasive properties. EMT has been suggested to be of major importance for the metastatic cascade by facilitating detachment from the primary tumors and invasion into the surrounding stroma. Cellular plasticity within tumors is further driven by a variety of differentiation states. These states resemble both stem/progenitor cells and the differentiated cell types of the organ where the tumor originated from. Cells with enhanced stem- or progenitor cell abilities exhibit the capacity to fuel the growth of tumors, while terminally differentiated cells within tumors have poor potential to drive tumor growth.
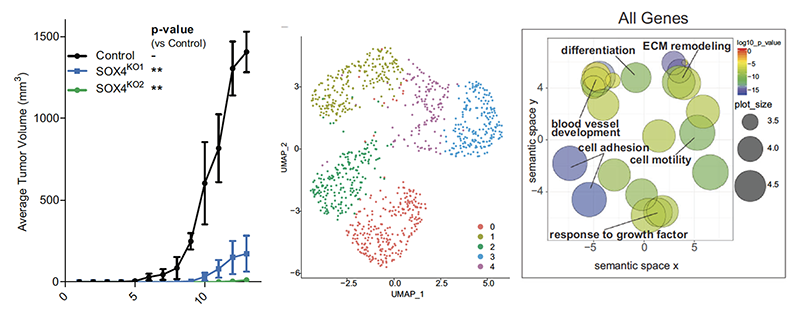
An increasing number of studies attest to an important role for the transcription factor SOX4 in cancer. Our own work has shown that SOX4 protein expression correlates with tumor size, mitotic index and poor prognosis of breast cancer patients. We and others have also shown a role for SOX4 in promoting EMT. However, until now the role of SOX4 in breast cancer has been studied in cell lines consisting of untransformed epithelial cells or in basal/mesenchymal-like tumor cells. Here we have interrogated the role of SOX4 both in vitro using breast tumor organoids and in vivo using a breast cancer mouse model.
In contrast to previous findings, we observed that deletion of SOX4 from tumors does not inhibit EMT in vivo. Instead, we found that SOX4 restrains differentiation by regulating expression of fetal mammary stem cell genes. Furthermore, SOX4 activates a cell-cycle gene expression program that shares gene sets with many progenitor cell types and primes the cells for proliferation in vivo. Consequently, loss of SOX4 leads to a strong impairment of tumor growth. Together, this study uncovers a novel mechanism by which SOX4 regulates a progenitor cell cycle program that is crucial for propagation in breast cancer. Therapeutic manipulation of SOX4 may thus provide a novel approach to interfere with tumor propagating cells.
This work was driven by Dr. Guy Roukens and was part of a great collaboration with Prof. Jacco van Rhenen’s lab. A long and difficult study full of surprises where our initial hypothesis was completely turned on its head. BIG thanks to everyone involved this really was a multi-group, team effort. You can read the full open access publication HERE.