Happy to be able to present our latest data on metabolism and autophagy in T cell activation and tolerance at the EMBO Cancer Immunometabolism meeting. https://meetings.embo.org/event/21-cancer
Tumor cells undergo metabolic changes to cope with the demands of rapid proliferation. But so do immune cells, which reprogram their metabolism when they encounter antigens and inflammatory signals. Drugs targeting cancer metabolism, as well as diets, could therefore also affect the immune system. Yet, to what extent cancer and immune cells respond in a similar fashion is currently unclear.
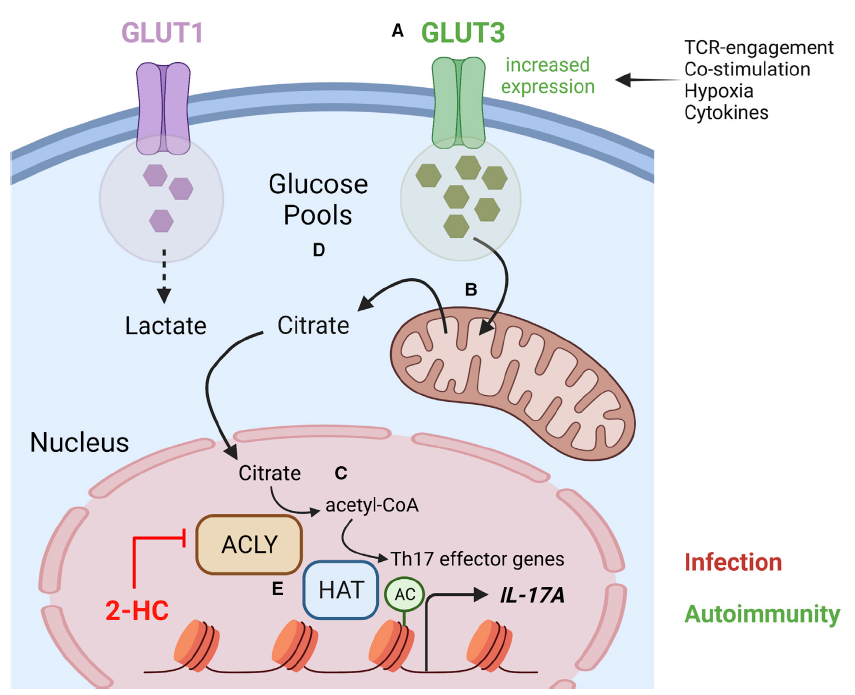
The immune system comprises specialized cell populations conditioned to respond rapidly to antigenic and inflammatory signals. Upon encountering antigen, the fate of CD4+ T helper cells is determined by multiple of signals they encounter which helps to shape the immune response. In addition to recognition of cognate antigen by the T cell receptor (TCR), CD4+ T cells integrate a myriad of additional cues, including signaling through costimulatory and coinhibitory receptors, interaction with other cell types, cytokines, or the type and availability of nutrients. Integration of these signals results in the induction of epigenetic and transcriptional programs driving proliferation, survival, and differentiation to T-effector cell subtypes. Changes in the activation status of CD4+ T lymphocytes not only require energy, but also increased demand for metabolic precursors for the biosynthesis of proteins, nucleic acids, and lipids. In the last decade, it has become clear that both autophagy and intracellular metabolism are essential for immune homeostasis. Productive TCR-engagement results in the induction of an unusual form of autophagy that is an absolute requirement for T cell activation. Failure to engage the autophagy machinery instead results in the induction of anergy, a hyporesponsive state. This is concomitant with a reduction in both activation-induced glycolysis and mitochondrial respiration. While it is clear that a glycolytic switch is also essential for productive T cell activation, the precise function of this remains unclear. We focus on how these processes impinge on the regulation of epigenetic and transcriptional changes required for CD4+ T cell activation and differentiation. Targeting the autophagy machinery and metabolism in the context of T cell activation may represent an effective approach to balance tolerance in immune cells.